TB Assay
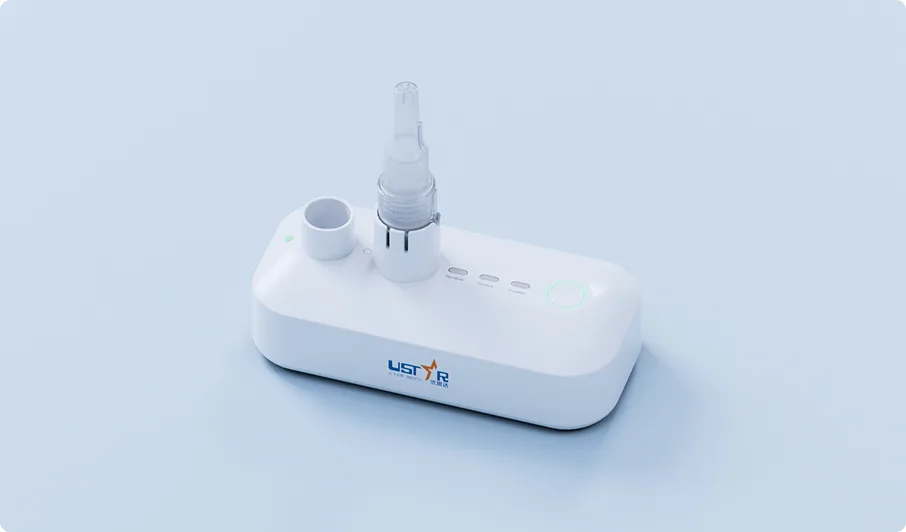
Product Overview
PortNAT TB Assay, conducted on Ustar’s PortNAT® system, ensures accurate results, simplifies the testing process, and offers the flexibility to deliver results when and where they are needed to meet timely testing demands.
Key benefits include:
- High-quality results within approximately 30 minutes
- Design to provide with a LOD: 400 CFU/mL
- Ability to perform testing anytime, anywhere
- Less than 1 minute of handling time, with no specimen preparation required
- Utilize a power bank for operation in locations without electrical supply
- For research use only
Ongoing Challanges
- In 2022, an estimated 10.6 million people worldwide developed tuberculosis, with an incidence rate of 133 per 100,000 population[1]
- In 2022, 6.2 million cases of pulmonary tuberculosis were diagnosed globally, of which 37% were not confirmed bacteriologically[1]
- TB caused 1.3 million deaths globally in 2022, nearly double the deaths caused by HIV/AIDS[1]
- Early and accurate detection of TB is essential for improving case management and significantly preventing transmission
[1]Global Tuberculosis Report
Spare Parts:
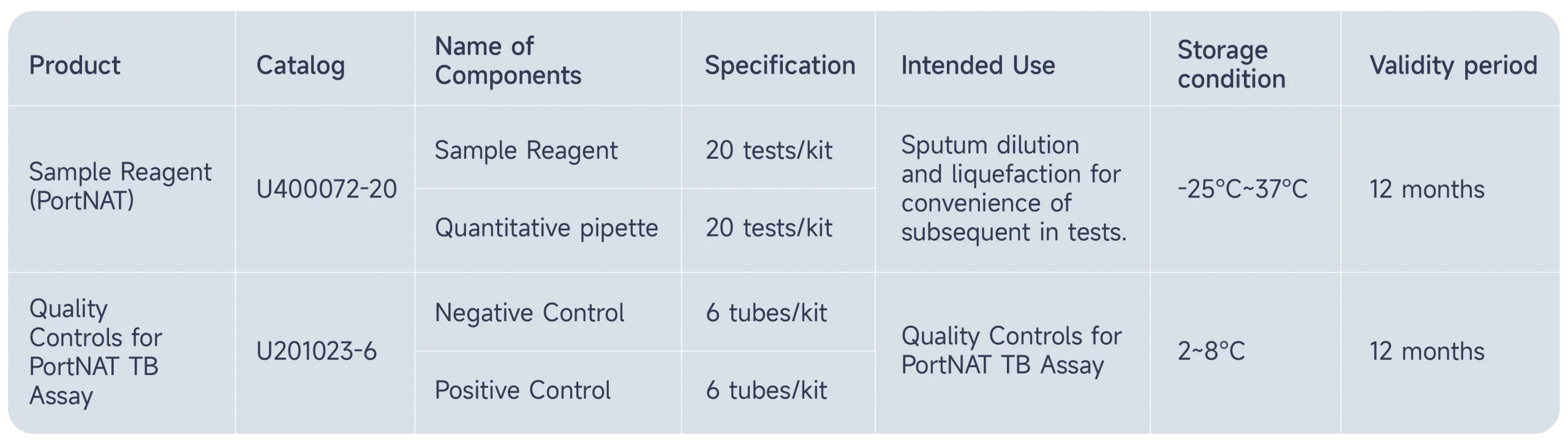
Product Resources
PortNAT® TB Assay
Brochure