Company Profile

Overview
Hangzhou Ustar Biotechnologies Co., Ltd. was founded in 2005 and has been dedicated to the research, development, production, and sales of innovative point-of-care molecular diagnostic (POCT) technologies and products.
Ustar has developed a comprehensive molecular POCT solution covering clinical diagnostics, diseases prevention and control, and agricultural and livestock quarantine. In the future, its reagent pipeline will be expanded from pathogen diagnostics to autoimmune diseases, tumor mutations, and pharmacogenetics
Ustar Technology



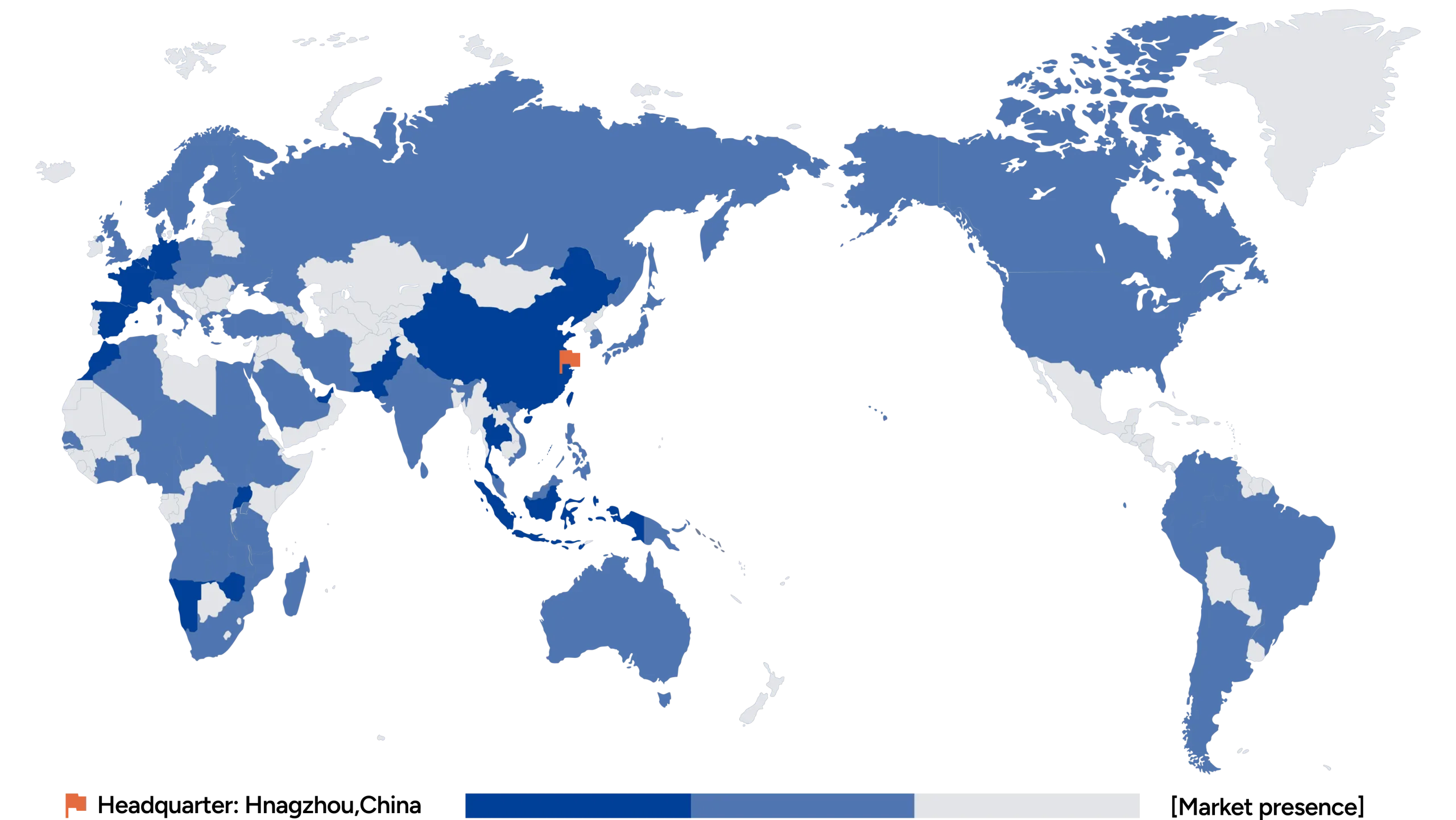
Global Recognition
The company has obtained NMPA Class III medical device certification for 2 devices and over 10 reagents, CE IVDR certification for over 10 reagents, and multiple devices and reagents have received ANVISA, TGA, HSA, and other international certifications. Notably, its PortNAT and EasyNAT molecular POCT monkeypox nucleic acid detection reagents have been included in the World Health Organization (WHO) Emergency Use Listing (EUL).
Enterprise Culture
Expanding innovation across the globle, delivering solutions worldwide.
Ustar’s products and technologies have served more than 3,000 medical institutions and have been exported to over 90 countries, actively contributing to global efforts in combating major infectious diseases such as tuberculosis.