Immediate Answer for Respiratory Infections
- Respiratory infectious diseases are among the most common clinical conditions. According to the World Health Organization’s (WHO) 2019 report on the top 10 global causes of death, lower respiratory tract infections ranked fourth, causing 2.6 million deaths. Respiratory infections often present with similar clinical symptoms, and since the COVID-19 pandemic, atypical presentations have become more frequent, making clinical differentiation challenging. High-risk groups such as children, pregnant women, and the elderly face a higher likelihood of severe illness and mortality when infected with respiratory pathogens.
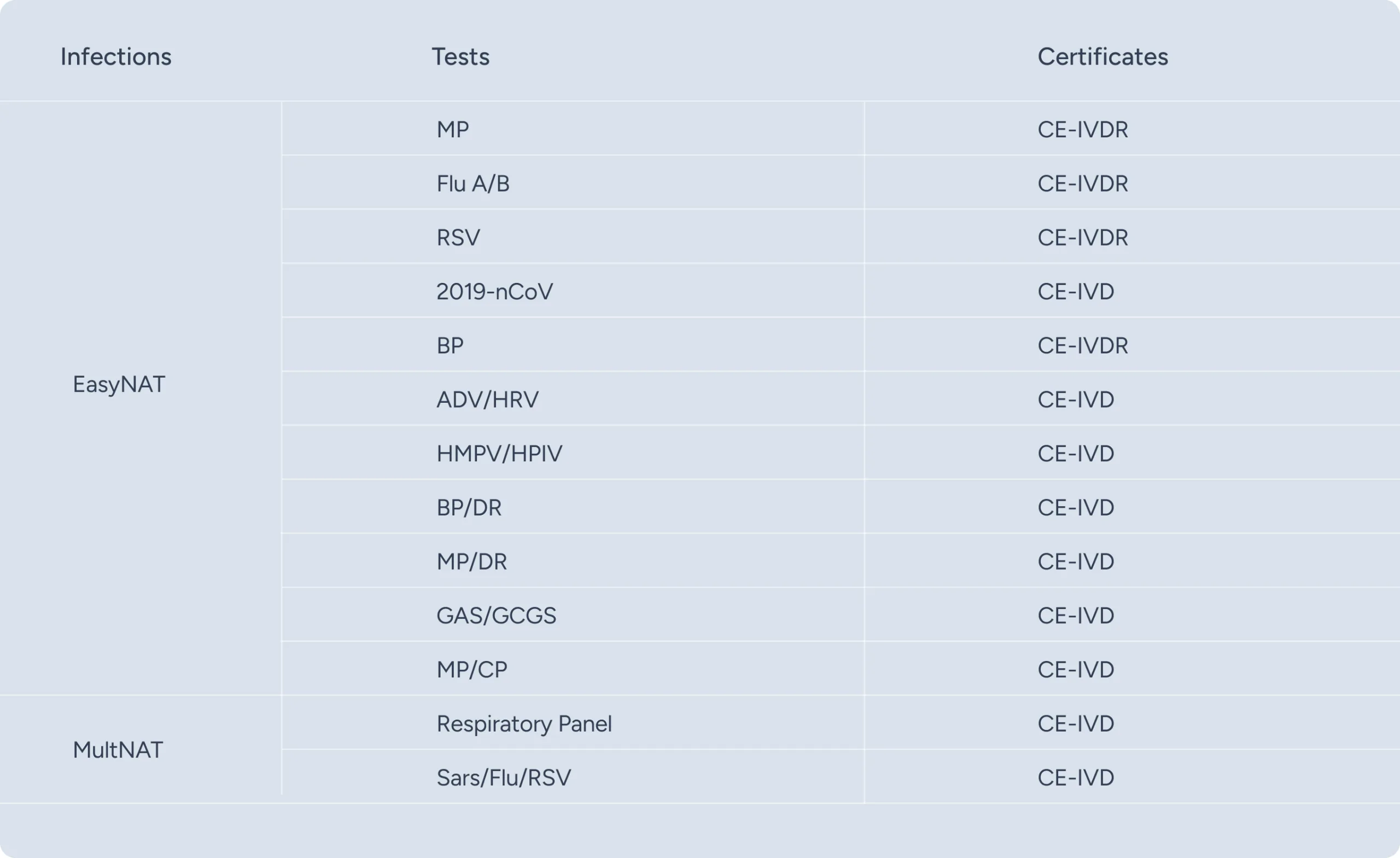
- The USTAR EasyNAT and MultNAT nucleic acid testing system enables fast and accurate identification of respiratory pathogens in clinical settings, guiding precise treatment decisions. With broad pathogen coverage (including Mycoplasma pneumoniae and Bordetella pertussis, which antigen tests cannot effectively detect), it holds significant clinical value in shortening disease duration, improving prognosis, and preventing severe complications.
No Specialized Laboratory Required:
The Expert Consensus on the Standardized Clinical Application of Rapid Pathogen Nucleic Acid Testing (2025 Edition) states that fully integrated and enclosed rapid pathogen nucleic acid testing can be conducted in a well-ventilated laboratory without the need for separate functional zones. The same laboratory can also perform routine blood tests, biochemical tests, and other assays.
Fast Turn-around-time (TAT):
Delivers results within 25 minutes, providing rapid clinical decision support.
Flexible Combinations:
Available in single and dual-target formats, enabling clinicians to adapt testing based on seasonal prevalence while reducing healthcare burden.
High Throughput:
Options of 4, 8, 16 or 32 tests per run to meet both routine standardized testing needs and urgent demands during peak periods.
On-Demand Testing:
Independent testing channels eliminate batch waiting, enhancing patient experience.
Head-to-Head Studies:
Conducted real-world clinical evaluations against Xpert® Xpress Flu/RSV at leading hospitals such as Huashan Hospital of Fudan University and the First Affiliated Hospital of Sun Yat-sen University, showing >99% concordance, with stable and reliable performance.
Strong Market Presence:
Exported to over 90 countries and serving more than 3,000 medical institutions in China, including 90+ top-tier hospitals.