R&D Strength
Our team
The company has a R&D team with more than 150 people, accounting for 32% of the total number of employees, of which more than 50% have master’s or above degree.
Innovation Awards
Vitrification Technology
The liquid enzyme is dehydrated and solidified into a “glassy state” without crystallization, which allows long-term storage in a low-oxygen, low-humidity environment and enables the transportation of reagents at room temperature.
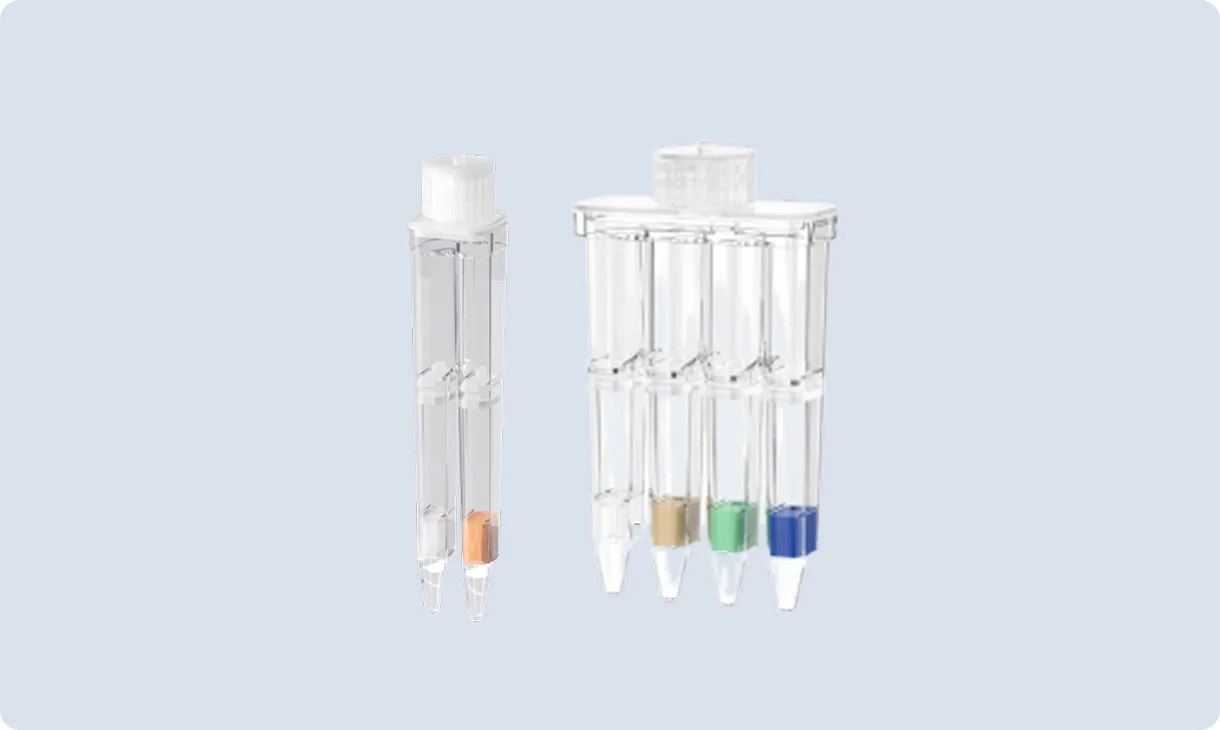
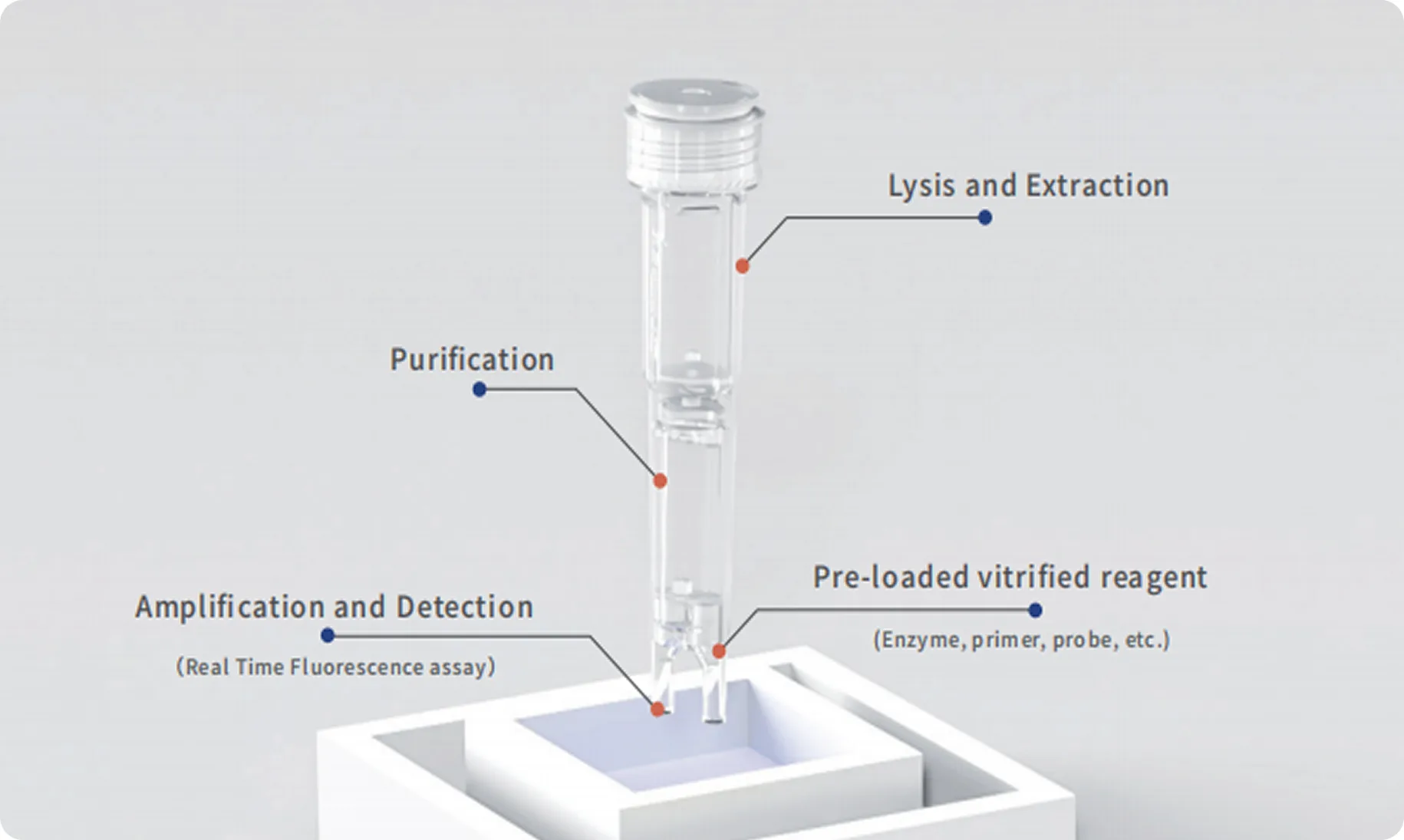
POCT Intergrated Tube
Integrating sample lysis, nucleic acid extraction and purification, amplification and detection in one tube, it realizes a fully enclosed nucleic acid testing process of “sample in – result out”, significantly reducing sample turnover time while avoiding aerosol contamination.

Disposable Nucleic Acid Detection Cartridge
Independently patented nucleic acid immunochromatography technology completes rapid analysis of nucleic acid amplicon in a closed cartridge, avoiding aerosol contamination and ensuring biosafety.
Clinical Evaluation and Academic Promotion
Published in reputable academic journals—Enhances the credibility and international influence of the products
A Clinical trial conducted in Dutch (KOL) Lab, shows a high level of agreement between the EasyNAT Malaria assay and the Alethia Malaria assay when screening returning travellers with suspected malaria in a non-endemic setting.

Clinical evaluation in some important countries
South Africa MultNAT MTC/RIF Clinical Trial: High sensitivity (Performance RlF resistance detection with sputum panel without/with sonication).
Nigeria EasyNAT Malaria Clinical Trial:Accuracy: 99%, Specificity: 98%, Sensitivity: 100%
Netherland EasyNAT Malaria Clinical Trial: Overall sensitivity and specificity were: 100% and 97.5%
Achievements
Quality System Certification


Won 10 international IVDR certificates + multiple IVDD certificates
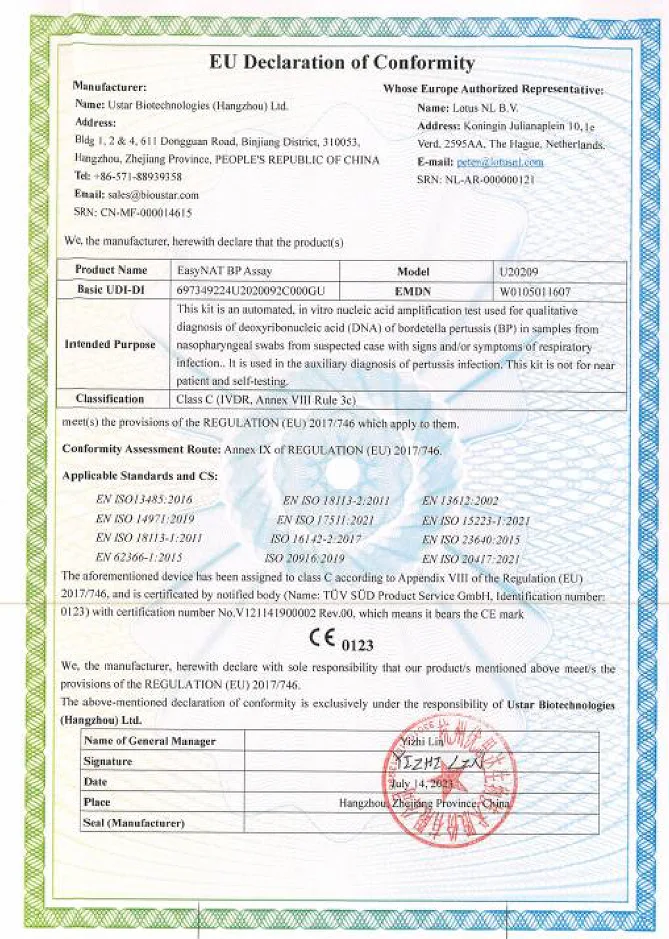
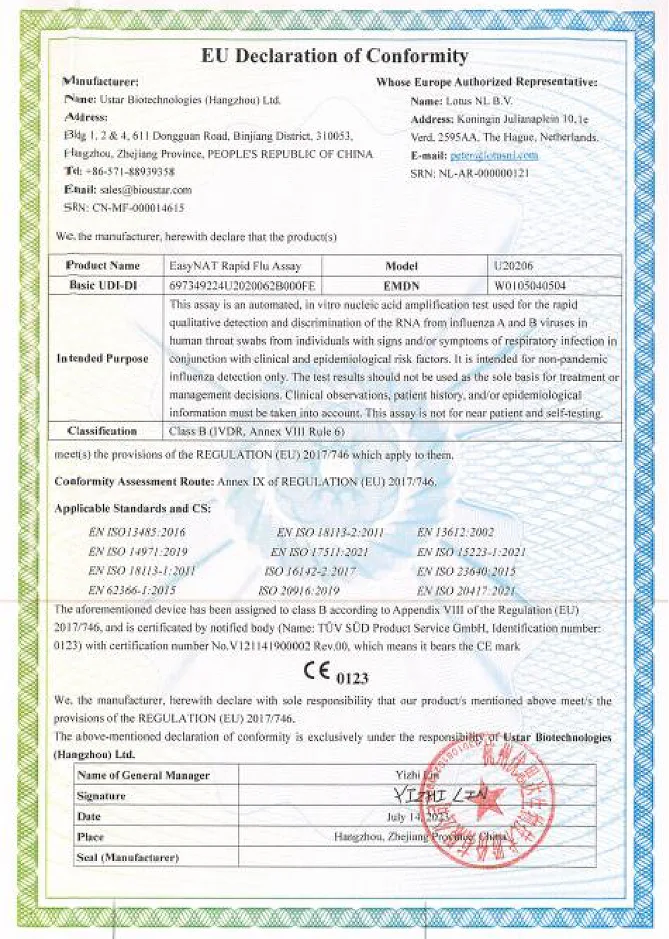
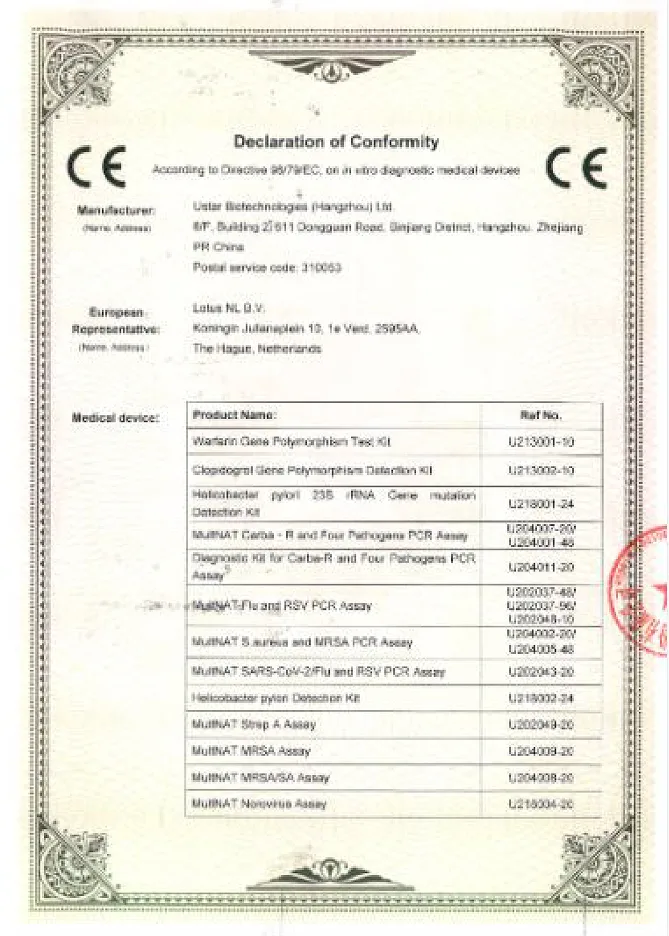

Global Registered Certificate Situation




More than 90 domestic and foreign patents have been applied, 62 have been authorized



