USTAR BIOTECHNOLOGIES
Molecular Testing,
Anywhere!
Ustar aligned with
Indonesia University to build a joint POC
Moleduclar innovation center
Solution for Rapid Nucleic Acid Detection Systems for Dengue, Zika and Chikungunya viruses
Ustar Biotechnologies’ Monkeypox Virus Assays Receive WHO Emergency Use Listing (EUL).
Systems and Reagents
EasyNAT Nucleic Acid Amplification and Detection Analyzer
Solution for Screening with Rapid and Accurate Results
MultNAT Molecular Diagnostic Testing System
For Multiplex, Drug Resistence and Quantatitive Testing
PortNAT Molecular Diagnostic Testing System
Designed for primary healthcare systems
Full Solutions for TB Diagnostics
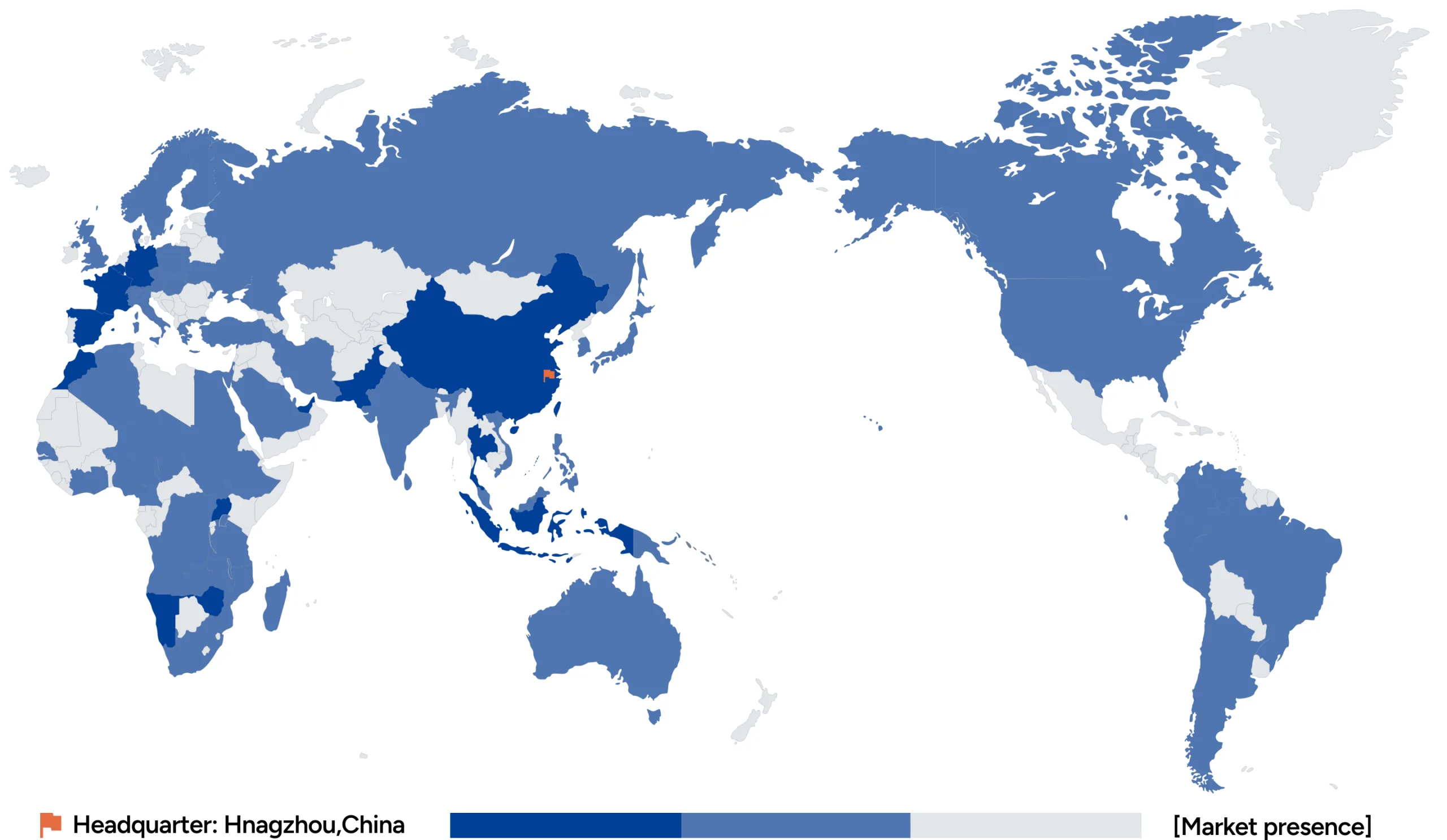
Ustar Biotechnologies
Ustar Biotechnologies (Hangzhou) Ltd. was established in 2005 and has been focused on the research, development, production, and sales of innovative point-of-care molecular diagnostic (POCT) technologies and products. The products and technologies have served over 3,000 medical institutions and are exported to more than 90 countries, actively contributing to the global control of major infectious diseases.



Expanding innovation across the globle, delivering solutions worldwide.