Monkeypox Virus Assay
Ongoing Challanges
- Given the ongoing expansion of the monkeypox epidemic in East and Central Africa, the number of cases reported in 2024 has already exceeded last year’s total, with more than 15,600 cases and 537 deaths[1] . Due to the potential for further spread both within and outside Africa, the World Health Organization (WHO) announced on August 14, 2024, that the monkeypox outbreak constitutes a “Public Health Emergency of International Concern” (PHEIC). This marks the second time since July 2022 that WHO has declared the monkeypox epidemic a PHEIC.
- The WHO has recommended nucleic acid amplification testing (NAAT) for viral DNA as the preferred laboratory diagnostic method for monkeypox.
[1]https://www.who.int/zh/news/item/14-08-2024-who-director-general-declares-mpox-outbreak-a-p
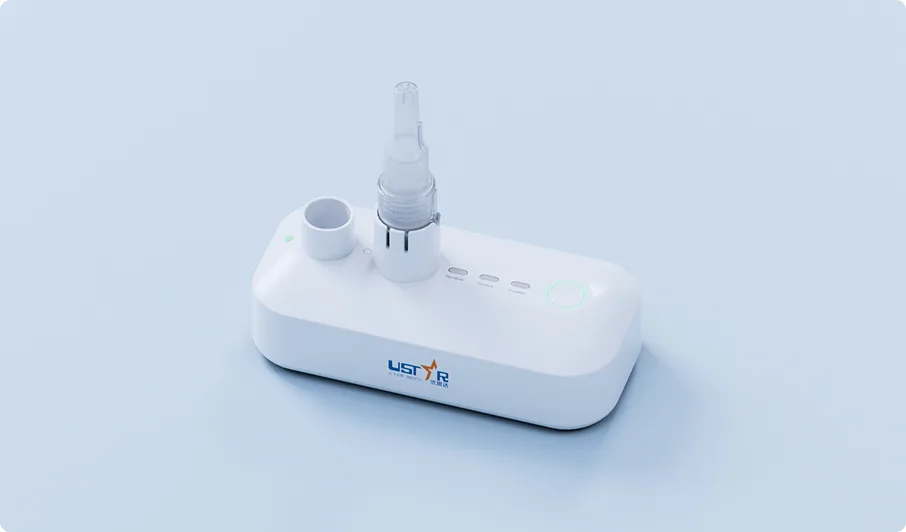
Product Overview
PortNAT Monkeypox Virus Test, conducted on Ustar’s PortNAT® system, ensures accurate results, simplifies the testing process, and offers the flexibility to deliver results when and where they are needed to meet timely testing demands. Key benefits include:
- High-quality results within approximately 40 minutes
- Design to provide a LOD: 500cps/ml
- Ability to perform testing anytime, anywhere
- Provides the convenience of on-demand testing with rapid and precise results.
Clinical Value
- Quickly identifying potential cases, helping to reduce the risk of community
- Speeding up patient diagnosis and treatment, enhancing workflow efficiency
- Providing timely guidance for vaccination and prevention strategy
Product Resources
EasyNAT® Monkeypox Virus Assay
Brochure
EasyNAT® Monkeypox Virus Assay
Instructions for Use
EasyNAT® Monkeypox Virus Assay
SOP