Respiratory Panel

Ongoing Challanges
- Respiratory targets pose significant challenges to human health due to their high transmissibility, ability to cause a wide range of diseases, and impact on vulnerable populations.
- Spread through droplets, aerosols, and contact, they can lead to acute and chronic conditions, exacerbate comorbidities, and overwhelm healthcare systems, particularly during pandemics such as influenza or COVID-19.
- Advanced diagnostics are crucial to mitigating their impact.
Product Overview
MultNAT® Respiratory Panel is a multiplexed nucleic acid test intended for the simultaneous qualitative detection and identification of multiple respiratory pathogen nucleic acids in nasopharyngeal swabs (NPS) collected from individuals suspected of respiratory tract infections.
- Streamlined process delivers precise Nucleic Acid Amplification Test (NAAT) results at 72 mins
- Point-of-care Nucleic Acid Testing applicable across diverse medical settings.
- Comprehensive detection of 24 distinct targets supports physicians in prescribing targeted treatments.
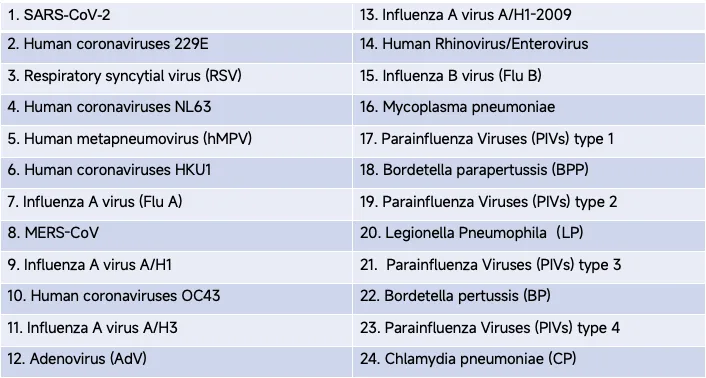
The following 24 respiratory targets are identified using this product:
Clinical Value
- The USTAR MultNAT Respiratory Panel detects and differentiates 24 targets of respiratory targets, including viruses and bacteria, offering broad diagnostic capabilities. This ensures the timely identification of the causative agent for respiratory infections.
- Differentiating between viral and bacterial targets helps reduce unnecessary antibiotic use and guides targeted therapy, improving patient outcomes and combating antimicrobial resistance.
- Rapid and reliable identification of targets enables physicians to initiate appropriate treatment, reduce complications, and minimize hospital stays, enhancing overall patient care.
Product Resources
MultNAT® Respiratory Panel
Brochure
MultNAT® Respiratory Panel
Instructions for Use
MultNAT® Respiratory Panel
SOP