Ustar Obtains the IVDR CE Certificate for 10 Products
- Categories:Latest News
- Author:
- Origin:
- Time of issue:2023-07-13
- Views:0
(Summary description)
Ustar Obtains the IVDR CE Certificate for 10 Products
(Summary description)
- Categories:Latest News
- Author:
- Origin:
- Time of issue:2023-07-13
- Views:0
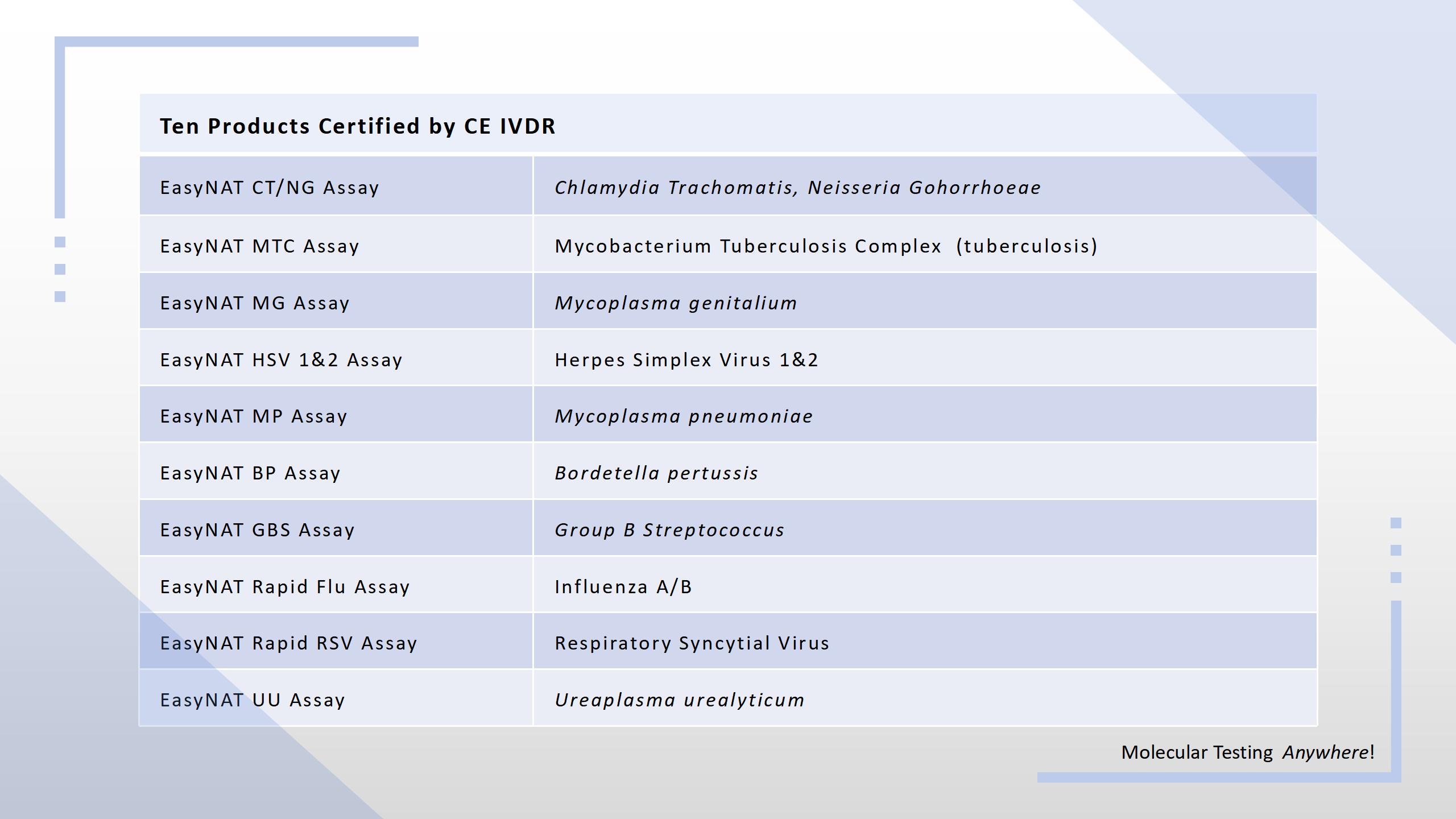
On July 11, Ustar received the IVDR CE certificate for 10 products from TÜV SÜD, a notified body designated under the EU's IVDR.
As one of the first IVDR-certified manufacturers for POC NAAT products in China, Ustar has made its important breakthroughs in the field of in vitro diagnostic medical devices, further consolidating its competitive advantage in the EU market.
 |
 |
The In Vitro Medical Devices Regulation (EU) 2017/746 (IVDR) is“the current regulatory basis for placing on the market, making available and putting into service in vitro diagnostic medical devices on the European market"[1]. It is intended to replace the existing EU In Vitro Diagnostic Medical Devices Directive (IVDD, Directive 98/79/EC). After the transition period, in vitro diagnostic medical devices without IVDR CE certification will no longer be able to enter the EU market. Obtaining IVDR CE certification is to ensure that the product complies with EU laws, regulations and standards, and reduces the risk of product sales in the European market, which is crucial for the sale of IVD medical devices in the European market.
The IVDR CE certificate issued to Ustar means that these ten products can be sold in Europe and other regions that accept EU certification. This greatly expands the market coverage, and provides strong support for further expanding the overseas customer base. As an IVD medical device company that meets strict safety standards and quality requirements, this certification will provide greater confidence and protection for the company's customers at home and abroad.
Reference:
[1]TÜV SÜD. UNDERSTANDING THE IN VITRO DIAGNOSTIC REGULATION (IVDR). Retrieved from https://www.tuvsud.com/en-us/industries/healthcare-and-medical-devices/medical-devices-and-ivd/medical-device-market-approval-and-certification/eu-in-vitro-diagnostic-medical-device-regulation
Scan the QR code to read on your phone
News

Contact us
Ustar Biotechnologies (Hangzhou) Ltd. (Headquarters)
Add: 6/F, Building 2, 611 Dongguan Road, Binjiang District, Hangzhou, Zhejiang, P.R. China
Zip: 310053
Email: sales@bioustar.com service@bioutsar.com
Tel: +86-571-88939358 (Sales)
+86-400-870-7025 (Service)
Copyright Ustar Biotechnologies 2018. All rights reserved 浙ICP备20029265号





