Clinical Data of Ustar EasyNAT MTC Assay Published in Frontiers in Microbiology
- Categories:Latest News
- Author:
- Origin:
- Time of issue:2022-04-28
- Views:0
(Summary description)A recent study published in Frontier in Microbiology proves that EasyNAT and Xpert methods show similar sensitivities and specificities.
Clinical Data of Ustar EasyNAT MTC Assay Published in Frontiers in Microbiology
(Summary description)A recent study published in Frontier in Microbiology proves that EasyNAT and Xpert methods show similar sensitivities and specificities.
- Categories:Latest News
- Author:
- Origin:
- Time of issue:2022-04-28
- Views:0
April 28, 2022 --- Low detection rates of Mycobacterium tuberculosis (MTB) by culture and smear microscopy prevent early diagnosis of tuberculosis (TB) in children. Therefore, developing rapid and accurate diagnostic techniques is critical to achieving the global aim of minimizing childhood TB.
A recent study, conducted by Beijing Children's Hospital, Capital Medical University (National Center for Children's Health, China), evaluates the diagnostic effectiveness of the novel cross-priming amplification-based Ustar EasyNAT MTC Assay in childhood TB.
The study tested gastric aspirate (GA) samples from children with suspected TB by bacterial culture, acid-fast bacillus microscopy, EasyNAT, Xpert MTB/RIF (Xpert), or Xpert MTB/RIF Ultra (Xpert Ultra) respectively.
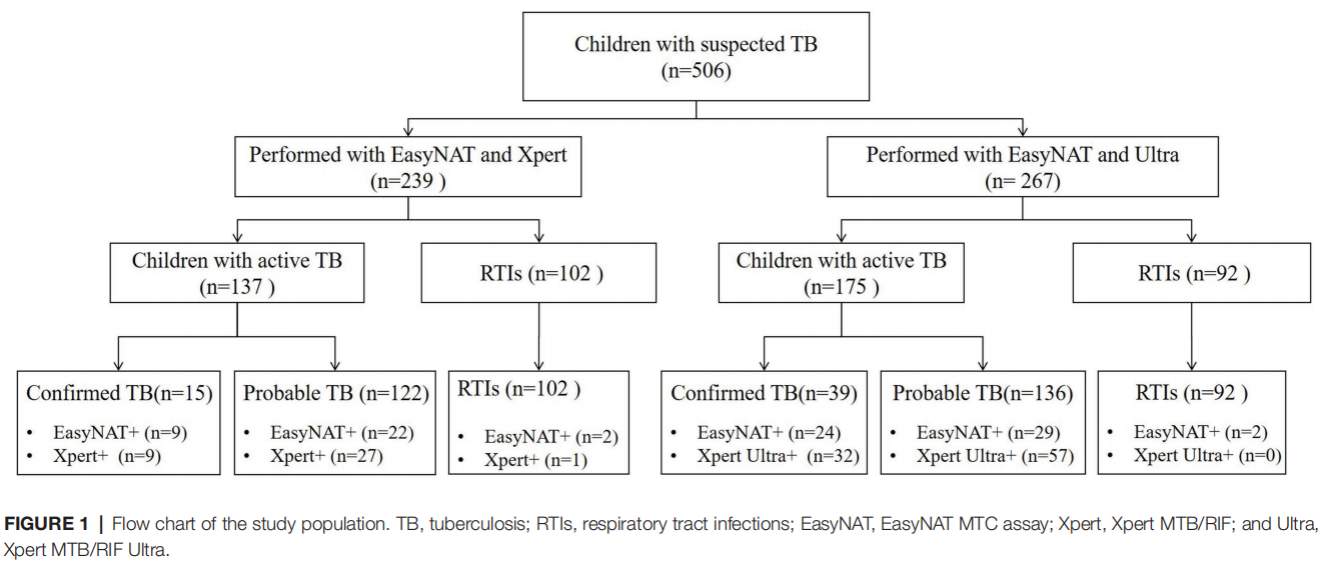
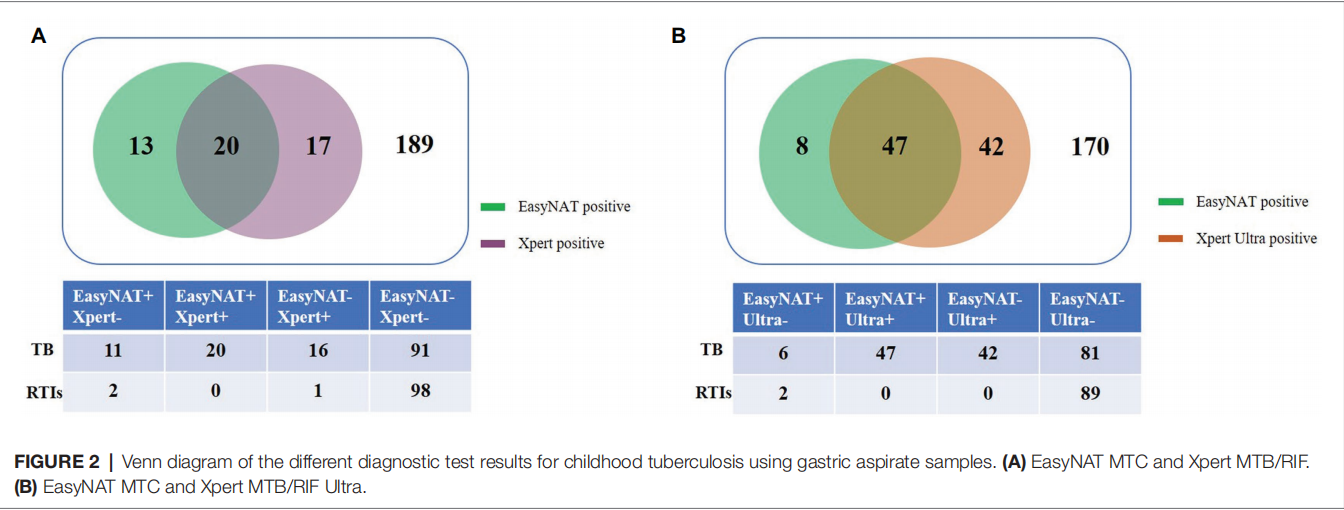
Results show that among 239 children simultaneously tested by EasyNAT and Xpert methods, both assays showed similar sensitivities in total active TB cases [22.6% (31/137) vs. 26.3% (36/137), p=0.441] and in bacteriologically confirmed TB cases [both 60.0% (9/15)]. The two assays presented similar specificities of 98.0% (100/102) and 99.0% (101/102), respectively (p=1.000).
In conclusion, EasyNAT assay has a similar diagnostic capacity for MTB in GA samples compared with the Xpert assay in children. EasyNAT may therefore be useful as a suitable alternative method of childhood TB diagnosis based on its cost-effectiveness, speed, and accuracy.
This study, titled A Novel Cross-Priming Amplification-Based Assay for Tuberculosis Diagnosis in Children Using Gastric Aspirate, was published in Frontiers in Microbiology, a leading international journal across the entire spectrum of microbiology.

Scan the QR code to read on your phone
News

Contact us
Ustar Biotechnologies (Hangzhou) Ltd. (Headquarters)
Add: 6/F, Building 2, 611 Dongguan Road, Binjiang District, Hangzhou, Zhejiang, P.R. China
Zip: 310053
Email: sales@bioustar.com service@bioutsar.com
Tel: +86-571-88939358 (Sales)
+86-400-870-7025 (Service)
Copyright Ustar Biotechnologies 2018. All rights reserved 浙ICP备20029265号





